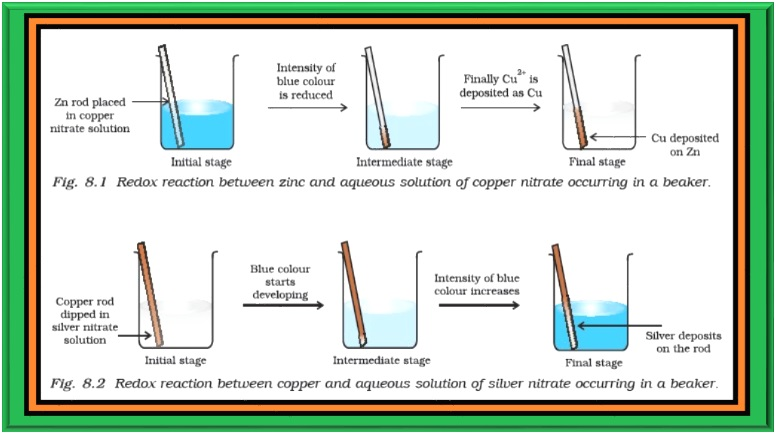
`=>` Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. 8.1, for about one hour. You may notice that the strip becomes coated with reddish metallic copper and the blue colour of the solution disappears.
`=>` Formation of `color{red}(Zn^(2+))` ions among the products can easily be judged when the blue colour of the solution due to `color{red}(Cu^(2+))` has disappeared.
`=>` If hydrogen sulphide gas is passed through the colourless solution containing `color{red}(Zn^(2+))` ions, appearance of white zinc sulphide, `color{red}(ZnS)` can be seen on making the solution alkaline with ammonia.
`=>` `color{green}("The reaction between metallic zinc and the aqueous solution of copper nitrate is :")`
`color{red}(Zn(s) + Cu^(2+) (aq) → Zn^(2+) (aq) + Cu(s))` ....................... (8.15)
`=>` In reaction (8.15), zinc has lost electrons to form `color{red}(Zn^(2+))` and, therefore, zinc is oxidised.
`=>` Evidently, now if zinc is oxidised, releasing electrons, something must be reduced, accepting the electrons lost by zinc. Copper
ion is reduced by gaining electrons from the zinc.
`=>` `color{green}("Reaction (8.15) may be rewritten as :")`

`=>` At this stage we may investigate the state of equilibrium for the reaction represented by equation (8.15).
`=>` For this purpose, let us place a strip of metallic copper in a zinc sulphate solution.
`=>` No visible reaction is noticed and attempt to detect the presence of `color{red}(Cu^(2+))` ions by passing `color{red}(H_2S)` gas through the solution to produce the black colour of cupric sulphide, `color{red}(CuS)`, does not succeed.
`=>` Cupric sulphide has such a low solubility that this is an extremely sensitive test; yet the amount of `color{red}(Cu^(2+))` formed cannot be detected. We thus conclude that the state of equilibrium for the reaction (8.15) greatly favours the products over the reactants
`=>` Let us extend electron transfer reaction now to copper metal and silver nitrate solution in water and arrange a set-up as shown in Fig. 8.2. The solution develops blue colour due to the formation of `color{red}(Cu^(2+))` ions on account of the reaction:

`=>` Here, `color{red}(Cu(s))` is oxidised to `color{red}(Cu^(2+)(aq))` and `color{red}(Ag^+(aq))` is reduced to `color{red}(Ag(s))`. Equilibrium greatly favours the products `color{red}(Cu^(2+) (aq))` and `color{red}(Ag(s))`.
`=>` By way of contrast, let us also compare the reaction of metallic cobalt placed in nickel sulphate solution. The reaction that occurs here is :

`=>` At equilibrium, chemical tests reveal that both `color{red}(Ni^(2+)(aq))` and `color{red}(Co^(2+)(aq))` are present at moderate concentrations. In this case, neither the reactants [`color{red}(Co(s))` and `color{red}(Ni^(2+)(aq))`] nor the products [`color{red}(Co^(2+)(aq))` and `color{red}(Ni (s))`] are greatly favoured
`=>` This competition for release of electrons incidently reminds us of the competition for release of protons among acids.
`=>` The similarity suggests that we might develop a table in which metals and their ions are listed on the basis of their tendency to release electrons just as we do in the case of acids to indicate the strength of the acids.
`=>` As a matter of fact we have already made certain comparisons.
By comparison we have come to know that zinc releases electrons to copper and copper releases electrons to silver and, therefore, the electron releasing tendency of the metals is in the order: `color{red}(Zn > Cu > Ag)`.
`=>` Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. 8.1, for about one hour. You may notice that the strip becomes coated with reddish metallic copper and the blue colour of the solution disappears.
`=>` Formation of `color{red}(Zn^(2+))` ions among the products can easily be judged when the blue colour of the solution due to `color{red}(Cu^(2+))` has disappeared.
`=>` If hydrogen sulphide gas is passed through the colourless solution containing `color{red}(Zn^(2+))` ions, appearance of white zinc sulphide, `color{red}(ZnS)` can be seen on making the solution alkaline with ammonia.
`=>` `color{green}("The reaction between metallic zinc and the aqueous solution of copper nitrate is :")`
`color{red}(Zn(s) + Cu^(2+) (aq) → Zn^(2+) (aq) + Cu(s))` ....................... (8.15)
`=>` In reaction (8.15), zinc has lost electrons to form `color{red}(Zn^(2+))` and, therefore, zinc is oxidised.
`=>` Evidently, now if zinc is oxidised, releasing electrons, something must be reduced, accepting the electrons lost by zinc. Copper
ion is reduced by gaining electrons from the zinc.
`=>` `color{green}("Reaction (8.15) may be rewritten as :")`

`=>` At this stage we may investigate the state of equilibrium for the reaction represented by equation (8.15).
`=>` For this purpose, let us place a strip of metallic copper in a zinc sulphate solution.
`=>` No visible reaction is noticed and attempt to detect the presence of `color{red}(Cu^(2+))` ions by passing `color{red}(H_2S)` gas through the solution to produce the black colour of cupric sulphide, `color{red}(CuS)`, does not succeed.
`=>` Cupric sulphide has such a low solubility that this is an extremely sensitive test; yet the amount of `color{red}(Cu^(2+))` formed cannot be detected. We thus conclude that the state of equilibrium for the reaction (8.15) greatly favours the products over the reactants
`=>` Let us extend electron transfer reaction now to copper metal and silver nitrate solution in water and arrange a set-up as shown in Fig. 8.2. The solution develops blue colour due to the formation of `color{red}(Cu^(2+))` ions on account of the reaction:

`=>` Here, `color{red}(Cu(s))` is oxidised to `color{red}(Cu^(2+)(aq))` and `color{red}(Ag^+(aq))` is reduced to `color{red}(Ag(s))`. Equilibrium greatly favours the products `color{red}(Cu^(2+) (aq))` and `color{red}(Ag(s))`.
`=>` By way of contrast, let us also compare the reaction of metallic cobalt placed in nickel sulphate solution. The reaction that occurs here is :

`=>` At equilibrium, chemical tests reveal that both `color{red}(Ni^(2+)(aq))` and `color{red}(Co^(2+)(aq))` are present at moderate concentrations. In this case, neither the reactants [`color{red}(Co(s))` and `color{red}(Ni^(2+)(aq))`] nor the products [`color{red}(Co^(2+)(aq))` and `color{red}(Ni (s))`] are greatly favoured
`=>` This competition for release of electrons incidently reminds us of the competition for release of protons among acids.
`=>` The similarity suggests that we might develop a table in which metals and their ions are listed on the basis of their tendency to release electrons just as we do in the case of acids to indicate the strength of the acids.
`=>` As a matter of fact we have already made certain comparisons.
By comparison we have come to know that zinc releases electrons to copper and copper releases electrons to silver and, therefore, the electron releasing tendency of the metals is in the order: `color{red}(Zn > Cu > Ag)`.
 In the reactions given below, identify the species undergoing oxidation and reduction :
In the reactions given below, identify the species undergoing oxidation and reduction :